|
hc8meifmdc|20005939267D|healthm_live|health_library|health_library_details|0xfdff85bd010000002401000001000700
| Overview |
Collagen is a type of protein. Fibrous in nature, it connects and supports other bodily tissues, such as skin, bone, tendons, muscles, and cartilage. It also supports the internal organs and is even present in teeth. There are more than 25 types of collagens that naturally occur in the body.
Collagen is one of the most plentiful proteins present in the bodies of mammals, including humans. In fact, it makes up about 25 percent of the total amount of proteins in the body. Some people refer to collagen as the glue that holds the body together. Without it, the body would, quite literally, fall apart. Possessing great tensile strength, collagen functions in a manner that is very different from many other types of proteins. For example, it can be found both inside and outside of cells. Collagen fibers are important in contributing to the external structure of cells. However, they are present on the inside of some cells as well.
Collagen works hand-in-hand with elastin in supporting the body’s tissues. Basically, it gives body tissues form and provides firmness and strength; elastin gives the same body tissues much need flexibility. This combination of collagen and elastin is very important in many parts of the body, including, but not limited to, the lungs, bones, and tendons. Even the blood vessels rely on both collagen and elastin.
Often, collagen is discussed in relation to the skin. It works with keratin to provide the skin with strength, flexibility, and resilience. As people age, however, collagen degradation occurs, leading to wrinkles. As such, it is an important substance for those looking for ways to fight the visible effects of aging on the skin. Some skincare professionals actually advise people on ways to stimulate the production of collagen in skin cells.
In addition to being so important in the body, collagen also has many medical uses. It is used in some cosmetic surgery procedures and is sold as a supplement created for joint mobility. It is even used in treating and managing serious burns. For this purpose, it is used in creating man-made skin substitutes.
Since collagens are so important within the body, it stands to reason that collagen deficiencies can be problematic. In fact, there are some genetic diseases that are associated with collagen deficiencies. For example, osteogenesis imperfecta, commonly referred to as brittle bone disease, results from a significantly decreased level of collagen. It can also result from the presence of collagen that is of lower quality than normal.
What is Collagen?
|
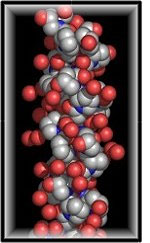 |
| Molecules of collagen exhibiting a triple helix structure |
| |
|
It is a protein but a very special one. Its structure is made up of amino acids with a fibrous arrangement, otherwise known as fibrous scleroproteins. The fibers are long chain molecules, containing up to 19 amino acids. The most important of these being, prolin, glicyn and hydroxyprolin and hydroxysilizin. The last two compounds are not found in any other proteins. Amino acids are in turn composed of the so called life elements, carbon, hydrogen, oxygen and nitrogen. The combination of a hundred or more of these elements in a specific pattern produces amino acids, which can then further combine to produce long polypeptide molecular chains. These long chains of amino acids co-join to form super helixes with a space volume structure. The whole chain structure relies on a weak hydrogen type bond for support. The most desirable property of these collagen super helixes is their sequence of three repeating amino acids which undertake a specific pattern, represented graphically as: _X_Y_Z_ Where, the Y and Z radicals are built into the polypeptide chain. These chains are the life form of a living organism and can be rendered apart during periods of illness; for instance in cases of pneumonia, where very high body temperatures can be encountered, in excess of 42 degrees Centigrade. |
 |
| Molecules of collagen showing structure disrupted structure by high temperatures |
 |
| A protein |
| |
Where is it Found?
About 20 types of collagen are present in living organisms. The most important ones are listed below, together with where they occur.
Collagen I- ligaments, bone, cornea, teeth, fibrous cartilage, womb and the inter - vertebral disc.
Collagen II-Cartilage, vitreous cells and intervertebral disc.
Collagen III-Womb, skin, intestines, heart membranes, jaws.
Collagen IV-Membranes.
Collagen V-Cornea, bone, cartilage.
Collagen VI-Stomach membrane, skin and cartilage.
Collagen VII-Skin, lungs, cornea.
Collagen VIII-Unknown, produced in body cells.
Collagen IX-Cartilage.
Collagen X-Produces chronodrocites during ossification.
Collagen XI-Cartilage, intervertebral cartilage and bone enamel.
Collagen XII-Ligaments, tendons and tooth enamel. |
| |
|
|
| |
|
Benefits / Uses
Collagen is the most powerful protein in our system. The name collagen is derived from Greek and means healing, bonding. Its highest quantities occur in the skeletal system, the skin, organ of sight, the kidneys, liver, alimentary tract. The extracellular fluid in which we are submerged consists of collagen, which flows around the organs, supplementing existing structural defects. Collagen is produced in the cells of connective tissues, fibroblasts and in chondrocytes of the bone tissue. Sexual hormones, the growth hormone and growth factors, adrenal cortex and thyroid hormones participate in its biosynthetic process. The turnover of collagen occurs throughout our lives. What has been worn out degrades and shortages are being replaced immediately, just as in the aging process degeneration dominates over its synthesis in the skeletal system. Its various functions in the body can be outlined as under:
|
 |
rejuvenate maturing skins |
|
|
 |
deeply moisturize and tone skin. |
|
|
 |
delay the ageing process, |
|
|
 |
clearly improve skin immunology, compactness and pigmentation |
|
|
 |
smooth mimic wrinkles |
|
|
 |
smooth scars, stretch marks and scalded skin |
|
|
 |
regenerate hair and nails: gray hair gets darker, brows and eye lashes start growing again, nails get harder and more elastic. |
|
|
 |
force the semipeptides and amino acids into the extra cellular skin sub layer |
|
|
 |
elasticise and soften hard skin |
|
|
 |
it is the best balsam for after shaving and deputation |
 |
deeply hydrate the skin enhancing its firmness | |
| |
| Supports in the treatment of: |
 |
paradontosis |
|
|
 |
allergies |
|
|
 |
juvenile acne and acne rosacea |
|
|
 |
dermatosis, psoriasis and dandruff |
|
|
 |
cellulite |
|
|
 |
disorders of sebaceous and sweat glands |
|
|
 |
alopecia |
|
|
 |
arthritis |
|
|
 |
effective reducing the effects of minor burns , grazes and knocks |
|
|
 |
effective reducing bruises and bed sores |
|
|
 |
preventing premature greying of hair |
|
|
 |
reducing varicose veins |
|
|
 |
soften old scar tissue and prevents formation of unsightly new scar tissue |
|
|
 |
soothing pain associated with bones, joints and rheumatism |
|
|
 |
helping with the process of bone formation |
|
|
 |
osteoporosis: a disease whose main characteristics is the fragility and brittleness of bones; collagen may supplement the deficiency of protein in bones and restore their density and durability; at the same time, it has the analgesic effect reducing swellings |
|
|
 |
helping prevent skin infections |
|
|
 |
helping prevent hair follicie enlargement and bursting of small blood vessels |
|
|
 |
helping in the healing of broken bones, sprains and after birth pains |
|
|
 |
regenerating the vaginal sheath |
|
|
 |
relieving parodontal pains |
|
|
 |
improving circulation of blood in arteries and veins |
|
|
 |
helping prevent patchy baldness. |
|
|
 |
enhancing visual acuity when applied on eye lids. |
 |
multiple sclerosis - this serious and so far incurable disease is characterized by a gradual atrophy of protein sheath of nerve fibers which basically consist of collagen. | |
| |
Organ of sight
Hydrated collagen can be found in lenses. Together with polysaccharides it forms the optic disc.
Natural Collagen enhances visual acuity when applied on eye-lids.
Hair
Collagen constitutes the supporting material of hair follicles. Collagen pigment is identical with hair pigment. It restore beauty to the hair - gray hair gets darker, brows and eye lashes start growing again.
Bones
Classic example - like skin - of type I collagen occurrence in human body. Together with calcium and phosphorus salts type I collagen constitutes the bone building material. It forms 95% of bone matrix. It undergoes all physiological changes, life biological cycles, diseases, and diets.
Nerves and vessels
Collagen is a component of nerve fiber myelin sheath, spinal cord, brain meninges, and nerve cell basement membranes. Vessels - arterial, venous, lymphatic.
Alimentary tract
Type II and III collagen is the scaffold supporting stomach, intestine and esophagus parietal cells.
Collagen in menopause
The decrease of collagen turnover in women in perimenopausal period causes a number of diseases of the bone system and genitals. In urinary-genital system the disturbances of genital and urinary tracts trophiecs and statics are most common.
Discussing collagen deficit in women in this period of their lives, C. Falconer stresses that commonly used hormone replacement therapy results in the increase of hydrated type II and III collagen store in the urinary-genital system. Falconer has also shown that a number of dysfunctions, e.g. genital organ lowering, mucous membrane dryness, urinary incontinence, and inflammatory states, are caused by the change of collagen quantity and quality. The lack or small production volume of type II and III collagen by connective tissue cells cause the deficit of hydrated collagen in extracellular space. In such case the supply of estrogens results in a dramatic improvement.
Dosage
Collagen must be taken on an empty stomach, with water, at least 2 hours after a meal (3 hours after a heavy meal). It should be taken as one of the last things you do before retiring for the night. Daily dosage should be two capsules taken with a glass of water. The collagen has to pass through the stomach and into the intestine without being "damaged" (broken down) by stomach acid. This is why the stomach MUST be EMPTY. If it is broken down along with food, it is then no longer collagen and so becomes totally ineffective.
Possible Side-Effects / Precautions / Possible Interactions
Research Studies / References
|
 |
Müller, Werner E. G. (2003). "The Origin of Metazoan Complexity: Porifera as Integrated Animals". Integrated Computational Biology 43 (1): 3-10. doi:10.1093/icb/43.1.3 |
|
|
 |
Di Lullo, Gloria A.; Sweeney, Shawn M.; Körkkö, Jarmo; Ala-Kokko, Leena; San Antonio, James D. (2002). "Mapping the Ligand-binding Sites and Disease-associated Mutations on the Most Abundant Protein in the Human, Type I Collagen". J. Biol. Chem. 277 (6): 4223-4231. PMID 11704682. |
|
|
 |
Sikorski, Zdzisław E. (2001). Chemical and Functional Properties of Food Proteins. Boca Raton: CRC Press. p. 242. ISBN 1566769604. |
|
|
 |
Wyckoff, R.; Corey, R.; Biscoe, J. (1935). "X-ray reflections of long spacing from tendon". Science 82 (2121): 175-176. doi:10.1126/science.82.2121.175. PMID 17810172. |
|
|
 |
Clark, G.; Parker, E.; Schaad, J.; Warren, W. J. (1935). "New measurements of previously unknown large interplanar spacings in natural materials". J. Amer. Chem. Soc 57 (8): 1509. doi:10.1021/ja01311a504. |
|
|
 |
"GNR - A Tribute - Resonance - October 2001". http://www.ias.ac.in/resonance/Oct2001/Oct2001p2-5.html. |
|
|
 |
Leonidas, Demetres D.; et al., GB; Jardine, AM; Li, S; Shapiro, R; Acharya, KR (2001). "Binding of Phosphate and pyrophosphate ions at the active site of human angiogenin as revealed by X-ray crystallography". Protein Science 10 (8): 1669-1676. doi:10.1110/ps.13601. PMID 11468363. |
|
|
 |
Subramanian, Easwara (2001). "Obituary: G.N. Ramachandran". Nature Structural & Molecular Biology 8 (6): 489-491. PMID 11373614. |
|
|
 |
Fraser, R. D.; MacRae, T. P.; Suzuki, E. (1979). "Chain conformation in the collagen molecule". J Mol Biol 129 (3): 463-481. doi:10.1016/0022-2836(79)90507-2. |
|
|
 |
Okuyama, K.; et al., K; Arnott, S; Takayanagi, M; Kakudo, M (1981). "Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)10". J Mol Biol 152 (2): 427-443. doi:10.1016/0022-2836(81)90252-7. PMID 7328660. |
|
|
 |
Traub, W.; Yonath, A.; Segal, D. M. (1969). "On the molecular structure of collagen". Nature 221 (5184): 914-917. doi:10.1038/221914a0. |
|
|
 |
Bella, J.; Eaton, M.; Brodsky, B.; Berman, H. M. (1994). "Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution". Science 266 (5182): 75-81. PMID 7695699. |
|
|
 |
Hulmes, D. J.; Miller, A. (1979). "Quasi-hexagonal molecular packing in collagen fibrils". Nature 282 (5741): 878-880. doi:10.1038/282878a0. PMID 514368. |
|
|
 |
esior, J. C.; Miller, A.; Berthet-Colominas, C. (1980). "Crystalline three-dimensional packing is general characteristic of type I collagen fibrils". FEBS Lett 113 (2): 238-240. doi:10.1016/0014-5793(80)80600-4. PMID 7389896. |
|
|
 |
Fraser, R. D. B.; MacRae, T. P. (1981). "Unit cell and molecular connectivity in tendon collagen". Int. J. Biol. Macromol. 3 (3): 193-200. doi:10.1016/0141-8130(81)90063-5. |
|
|
 |
Fraser, R. D.; MacRae, T. P.; Miller, A. (1987). "Molecular packing in type I collagen fibrils". J Mol Biol 193 (1): 115-125. doi:10.1016/0022-2836(87)90631-0. PMID 3586015. |
|
|
 |
Wess, T. J.; et al., AP; Wess, L; Miller, A (1998). "Molecular packing of type I collagen in tendon". J Mol Biol 275 (2): 255-267. doi:10.1006/jmbi.1997.1449. PMID 9466908. |
|
|
 |
Raspanti, M.; Ottani, V.; Ruggeri, A. (1990). "Subfibrillar architecture and functional properties of collagen: a comparative study in rat tendons". J Anat. 172: 157-164. |
|
|
 |
Holmes, D. F.; Gilpin, C. J.; Baldock, C.; Ziese, U.; Koster, A. J.; Kadler, K. E. (2001). "Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization". PNAS 98 (13): 7307-7312. doi:10.1073/pnas.111150598. PMID 11390960. |
|
|
 |
Holmes, D. F.; Kadler, KE (2006). "The 10+4 microfibril structure of thin cartilage fibrils". PNAS 103 (46): 17249-17254. doi:10.1073/pnas.0608417103. PMID 17088555. |
|
|
 |
Orgel, J. P.; et al., TC; Miller, A; Wess, TJ (2006). "Microfibrillar structure of type I collagen in situ". PNAS 103 (24): 9001-9005. doi:10.1073/pnas.0502718103. PMID 16751282. |
|
|
 |
Hulmes, D. J. (2002). "Building collagen molecules, fibrils, and suprafibrillar structures". J Struct Biol 137 (1-2): 2-10. PMID 12064927. |
|
|
 |
Hulmes, D. J. (1992). "The collagen superfamily-diverse structures and assemblies". Essays Biochem 27: 49-67. PMID 1425603. |
|
|
 |
Perumal, S.; Antipova, O.; Orgel, J. P. (2008). "Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis". PNAS 105 (8): 2824-2829. doi:10.1073/pnas.0710588105. PMID 18287018. |
|
|
 |
Sweeney, S. M.; et al., JP; Fertala, A; McAuliffe, JD; Turner, KR; Di Lullo, GA; Chen, S; Antipova, O et al. (2008). "Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates". J Biol Chem 283 (30): 21187-21197. doi:10.1074/jbc.M709319200. PMID 18487200. |
|
|
 |
Twardowski, T.; et al., A.; Orgel, J. P.R.O.; San Antonio, J. D. (2007). "Type I collagen and collagen mimetics as angiogenesis promoting superpolymers". Curr Pharm Des 13 (35): 3608-3621. doi:10.2174/138161207782794176. http://www.ingentaconnect.com/content/ben/cpd/2007/00000013/00000035/art00009. |
|
|
 |
Sabiston textbook of surgery board review, 7th edition. Chapter 5 wound healing, question 14 |
|
|
 |
Söderhäll, C.; Marenholz, I.; Kerscher, T.; Rüschendorf, F; Rüschendorf, F.; Esparza-Gordillo, J.; et al., C; Mayr, G et al. (2007). "Variants in a Novel Epidermal Collagen Gene (COL29A1) Are Associated with Atopic Dermatitis". PLoS Biology 5 (9): e242. PMID 17850181. |
|
|
 |
Houck, J. C.; Sharma, V. K.; Patel, Y. M.; Gladner, J. A. (1968). "Induction of Collagenolytic and Proteolytic Activities by AntiInflammatory Drugs in the Skin and Fibroblasts". Biochemical Pharmacology 17 (10): 2081-2090. doi:10.1016/0006-2952(68)90182-2. PMID 4301453. |
|
|
 |
Al-Hadithy, H.; et al., DA; Addison, IE; Goldstone, AH; Snaith, ML (1982). "Neutrophil function in systemic lupus erythematosus and other collagen diseases". Ann Rheum Dis 41 (1): 33-38. PMID 7065727. |
|
|
 |
Fratzl, P. (2008). Collagen: Structure and Mechanics. New York: Springer. ISBN 038773905X. |
|
|
 |
Buehler, M. J. (2006). "Nature designs tough collagen: Explaining the nanostructure of collagen fibrils". PNAS 103 (33): 12285-12290. doi:10.1073/pnas.0603216103. PMID 16895989. |
|
|
 |
"Gelatin's Advantages: Health, Nutrition and Safety". http://www.gmap-gelatin.com/gelatin_adv.html. |
|
|
 |
Walker, Amélie A. (May 21, 1998). "Oldest Glue Discovered". Archaeolgy. http://www.archaeology.org/online/news/glue.html. |
|
|
 |
Ennker, I. C.; et al., JüRgen; Schoon, Doris; Schoon, Heinz Adolf; Rimpler, Manfred; Hetzer, Roland (1994). "Formaldehyde-free collagen glue in experimental lung gluing". Ann Thorac Surg. 57 (6): 1622-1627. doi:10.1016/0003-4975(94)90136-8. http://ats.ctsnetjournals.org/cgi/content/abstract/57/6/1622. |
|
|
 |
"Hydrolyzed Collagen pills usages". http://www.articlecat.com/Article/Hydrolyzed-Collagen--Protein-Hydrate/21273. |
|
|
 |
"www.articlesbase.com". http://www.articlesbase.com/skin-care-articles/can-collagen-be-absorbed-into-the-skin-or-is-it-all-just-one-big-hoax-674325.html.[dead link] |
|
|
 |
Blow, Nathan (2009). "Cell culture: building a better matrix". Nature Methods 6 (8): 619-622. doi:10.1038/nmeth0809-619. |
|
|
 |
http://facstaff.gpc.edu/'pgore/geology/geo102/cambrian.htm |
|
|
 |
We, K. M. T. O. (1996). "Fossil preservation in the Burgess Shale". Lethaia 29: 107-108. doi:10.1111/j.1502-3931.1996.tb01844.x. edit | |
| | |
|