|
hc8meifmdc|20005939267D|healthm_live|health_library|health_library_details|0xfdffa1bd010000006e01000001001800
| How Carnosine Protects Against Age-Related Disease |
| By Edward R. Rosick, DO, MPH, MS |
|
Proteins are the building blocks of life. Comprising amino acid chains, proteins serve both structural and functional roles within the human body. Structural proteins such as collagen give support to bones, tendons, and skin, while functional proteins known as enzymes catalyze life-sustaining biochemical reactions throughout the body.
As we age, however, these critical proteins are endangered by the damaging process known as glycation. Defined as a non-enzymatic reaction between proteins and sugars, glycation irreversibly alters the configuration of proteins. These altered proteins, known as advanced glycation end products (AGEs), can no longer effectively fulfill their critical roles throughout the body. AGEs have been implicated in many of the diseases associated with aging, including Alzheimer's, cancer, and heart disease. |
 |
Fortunately, a powerful nutrient called carnosine helps defend the body's proteins against the crippling effects of glycation. By preventing the formation of dangerous AGEs, carnosine may help the body's proteins retain their youthful vigor and function. Moreover, studies demonstrate that carnosine is also a powerful antioxidant. Carnosine's age-defying effects make this critical nutrient an essential cornerstone of every anti-aging program.
AGEs, Aging, and Free Radicals
Many people, especially those well versed in anti-aging medicine, are knowledgeable about free radicals. Far fewer are familiar with advanced glycation end products, or AGEs, molecules that may be just as important as free radicals in initiating the pathological processes associated with aging. AGEs are substances formed in the human body by the biochemical interaction between carbohydrates and proteins in a process known as the Maillard reaction.
|
 |
|
Interestingly enough, the Maillard reaction was first noted during the heating of foods in the presence of sugars; it is what gives cooked foods their unique texture, taste, and smell. One author of a study of AGEs made the following analogy: "the human body might be viewed as an extraordinary complex mixture of chemicals reacting in a low temperature (37° C) oven with a 76-year cooking cycle. Under these conditions, non-enzymatic reactions between carbohydrates and proteins, known collectively as Maillard or browning reactions, produce a wide range of age-related chemical modifications and cross-links in tissue proteins."1 This cross-linking of proteins with carbohydrates can have wide-reaching effects, as AGEs are known to have deleterious effects on the structural and functional properties of proteins and the tissues in which these proteins reside. When you consider that proteins are present everywhere in the human body, the destructive capability of AGEs becomes quite clear.
While AGEs are destructive in their own right, their interplay with free radicals causes even more havoc in the aging human body. Researchers now postulate that oxidative stress may be involved in AGE formation and that, in a vicious cycle, AGEs may induce even more oxidative stress. In fact, most AGEs that accumulate in proteins are produced under oxidative conditions. As these AGEs and free radicals accumulate in cells and tissues, molecular damage and degradation down to the level of DNA increases, leading to many of the conditions associated with growing old. A growing body of sound scientific evidence theorizes that AGEs and similar molecules such as advanced lipoxidation end products, or ALEs (the products of lipids cross-linking with sugars), are significant contributors to many common pathological processes leading to conditions such as Alzheimer's, cancer, and heart disease.2-7
Implicated in Alzheimer's
If you ask aging adults which disease they fear most, Alzheimer's disease is likely to be at the top of the list, and for good reason: this devastating neurological condition slowly but inexorably destroys people's ability to think while robbing them of their memory. Alzheimer's is the most common cause of dementia in those aged 65 or older, and current statistics indicate that this debilitating condition affects more than 15 million people worldwide. With America's rapidly aging population—an estimated 30% of the US population will be 65 or older by the year 2050—it is projected that 14 million people in the US alone will be affected by Alzheimer's disease in the next few decades.
While researchers have not been able to pinpoint the exact cause of Alzheimer's disease, it has been proposed that AGEs and oxidative damage participate in its genesis.8-11 Some early research showed that two brain tissue hallmarks of Alzheimer's—beta-amyloid plaques and neurofibrillary tangles—both contain significant amounts of AGEs.8 Beta-amyloid plaques are especially insidious in that they not only cause direct destruction of brain neurons, but also increase oxidative stress, which itself can cause neuronal death.
In a recent review article that examined the role of AGEs and oxidative stress in Alzheimer's disease, the authors showed that AGEs are present in higher amounts in the biopsied brains of patients who died from Alzheimer's than in the brains of unaffected individuals.11 They also presented experimental evidence that AGE formation in the brains of Alzheimer's patients occurs early in the disease process. The authors concluded, "our findings emphasize the importance of glycoxidation, AGE distribution, and oxidative stress in the pathogenesis of AD [Alzheimer's dementia] and further indicate that AGE formation represents an early event in disease development."
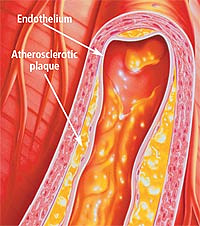
AGES and Arterial Plaque
Once the heart starts beating at approximately 16 weeks of age (as a fetus), it continues to beat every second of every day for the rest of a person's life. For someone who lives to be 80, that comes out to be roughly 2.5 trillion beats. Unfortunately, conditions that adversely affect this remarkable organ cause more death than any other type of disease. According to the latest figures, more than 60 million Americans suffer from some form of cardiovascular disease, such as coronary artery disease, and 7 million people will suffer a heart attack this year. More than 600,000 Americans will die this year from complications of coronary artery disease, with women accounting for 49% of those deaths.
Many factors contribute to coronary artery disease, including endothelial dysfunction, abnormal lipid profiles, and hypertension. (See also "Why Our Arteries Become Clogged as We Age," Life Extension, October 2005.) Emerging evidence suggests that AGEs are important components in the formation of potentially lethal atherosclerotic plaque, a sticky, hard substance that can significantly impede arterial blood flow.12-15
In a recent report, researchers critically examined the role of oxidation and AGEs in the development of atherosclerosis in both in-vitro (in the laboratory) and in-situ (in its original place) experiments.13 One of the crucial first steps in the development of coronary artery disease is an inflammatory response in the heart vessel walls. The researchers found that "CML [carboxymethyllysine, a type of AGE] is formed concomitantly with oxidative stress . . . and can be regarded as a biomarker for a low-grade inflammatory tissue reaction in the atherosclerotic plaque. Its formation via lipid oxidation may be involved in the development of atherosclerosis."
Possible Role in Cancer
In the last 35 years, the US government has spent almost $50 billion fighting the so-called "war on cancer." Its efforts, however, have failed to defeat this modern-day scourge. Approximately 1.3 million Americans will be diagnosed with cancer this year, and nearly 600,000 will die of various forms of the disease. One in every two American men and one in three women will eventually develop cancer. Despite billions of dollars spent on research and new cancer-fighting drugs that can cost patients thousands of dollars per week, the epidemic of cancer continues unabated.
Many wonder why the government, with its almost unlimited resources, has been unable to find a cure. One answer is that cancer, much like Alzheimer's disease, is a multifactorial illness. Unlike an infectious disease such as polio or smallpox, cancer is thought to be caused by various biochemical aberrations. Growing evidence suggests that damage caused by both free radicals and AGEs is part of the biochemical milieu that contributes to cancer.16-20
In the late 1990s, scientists used human pancreatic cells in culture to examine the role of AGEs in the initiation and development of pancreatic cancer.19 Their findings suggest that AGEs may "play an active part in the progression of pancreatic cancer." A more recent article in 2004 looked at the possible involvement of AGEs in the development and progression of melanoma.18 The incidence of this deadly disease, the most highly invasive and metastatic of all skin cancers, is rapidly growing. Using human cells, researchers found evidence that AGEs might be involved not only in the growth of melanoma, but also in its spread to other locations, or
|
| |
|
Carnosine's Protective Effects
Inhibiting the formation of AGEs and associated molecules is thus an essential part of any anti-aging protocol. According to a paper on how AGEs affect aging, "Inhibition of AGE/ALE formation is a reasonable target for life-span extension for several reasons. First, if damage to protein reflects damage to DNA, then inhibition of AGE/ALE formation . . . should limit damage and mutation in DNA, leading to an increase in maximum life span. Second, accumulation of AGE/ALEs in proteins is associated with a number of age-related, chronic diseases . . . inhibition of AGE/ALE formation might delay the progression pathology in these diseases, thereby improving the quality of life in old age . . . A third consideration is that inhibition of AGE/ALE formation might also limit secondary oxidative damage to biomolecules."1 |
 |
While several promising experimental drugs are being used to combat AGEs, none of these is available to the general public. Fortunately, aging adults have ready access to a unique nutrient—carnosine—that protects against the ravages of oxidative damage while inhibiting the formation of AGEs.
Carnosine is a safe, well-tolerated compound comprising the amino acids beta-alanine and L-histidine. Naturally present in high concentrations in human brain and skeletal muscle tissue, carnosine has been shown in multiple studies to inhibit lipid peroxidation and free radical-induced cellular damage. Additional evidence suggests that carnosine may help protect the brain against oxygen deprivation,21 delay the impairment of eyesight with aging,22 and extend the life span of mammals.23 In addition, scientists have now shown that carnosine can effectively inhibit AGE formation and protein cross-linking.10, 24-27 Because it is able to both stop the oxidative damage of free radicals and inhibit AGE formation, scientists have postulated that carnosine may have applications in the management of numerous conditions, including arthritis, stomach and duodenal ulcers, high blood pressure, adrenal cortical dysfunction, sleep apnea, chronic inflammation, cancer, heart disease, and Alzheimer's disease.
Staving Off Neurodegeneration
Currently available pharmaceutical medications prescribed for Alzheimer's disease do nothing to combat the damage caused by amyloid beta or oxidative stress, two key factors in the genesis of this devastating illness. Experimental studies, however, suggest that carnosine can help protect against both. By protecting the brain against free radical and AGE-induced damage, carnosine may provide a way to treat and manage Alzheimer's disease.10,28-30
One early study examined the ways in which carnosine may protect the brain against the toxic effects of malondialdehyde, an end product of lipid peroxidation.29 Using cultured rat brain cells, researchers showed that carnosine not only protected the brain cells against malondialdehyde-induced toxicity, but also inhibited malondialdehyde-induced protein cross-linking.
More recently, researchers examined carnosine's protective effects against amyloid beta.30 Using cultured rat brain cells, they showed that introducing amyloid beta to the culture caused significant toxic effects. The researchers then showed that damage to the brain cells could be substantially decreased by adding carnosine to the mixture. "We postulate that the mechanism of carnosine protection [of brain cells] lies in its anti-glycating and antioxidant activities, both of which are implicated in neuronal and endothelial cell damage during Alzheimer's disease," the researchers noted. "Carnosine may therefore be a useful therapeutic agent."
|
| |
|
Combating Heart Disease
Americans spend billions each year on expensive drugs designed to ward off and treat the secondary effects of atherosclerosis and coronary artery disease. Unfortunately, these costly, doctor-prescribed medications do nothing to prevent cardiac damage induced by AGEs and free radicals.
Multiple lines of study now indicate that carnosine may be a highly beneficial nutrient for people with heart disease.12,14,31,32 In a review article examining the role of free radicals and AGEs in atherosclerosis, researchers carefully outlined the ways in which oxidative damage and AGE toxicity can contribute to the formation of atherosclerotic plaques, a hallmark of heart disease.12 They then examined ways to impede plaque formation, concluding, "AGE inhibitors . . . will also inhibit the chemical modifications of proteins during lipid peroxidation reactions, and will prove useful in the treatment of atherosclerosis."
The theory that AGE inhibitors such as carnosine may be a useful adjunct in both preventing and treating heart disease has been borne out in animal research as well. In two studies using dogs, researchers showed that AGE-induced changes led to decreased heart function by contributing to collagen cross-linking.14,32 When this happens, heart blood vessels, as well as the heart muscle itself, lose elasticity and become less efficient. When old dogs received an AGE inhibitor, they demonstrated a marked decrease in heart muscle stiffness as well as improved overall cardiac function. |
 |
|
Conclusion
By protecting against both free radical-generated oxidative damage and AGE-generated cellular toxicity, carnosine helps to counteract numerous, potentially harmful biochemical processes associated with aging. Its diverse effects offer support for the aging brain and cardiovascular system, and may help to modulate processes that contribute to cancer. Carnosine's remarkable spectrum of health benefits makes this versatile nutrient an essential component of any anti-aging program. |
| |
| References |
|
|
 |
Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001 Sep;36(9):1527-37. |
|
|
 |
DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004 Jun;4(3):301-5. |
|
|
 |
Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002 Jun;1(3):465-79. |
|
|
 |
Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J Biol Chem. 2000 Jul 14;275(28):21177-84. |
|
|
 |
Vlassara H. Advanced glycation in health and disease: role of the modern environment. Ann NY Acad Sci. 2005 Jun;1043:452-60. |
|
|
 |
Baynes JW. The Maillard hypothesis on aging: time to focus on DNA. Ann NY Acad Sci. 2002 Apr;959:360-7. |
|
|
 |
Takeuchi M, Yamagishi S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med Hypotheses. 2004;63(3):449-52. |
|
|
 |
Munch G, Schinzel R, Loske C, et al. Alzheimer's disease—synergistic effects of glucose deficit, oxidative stress and advanced glycation endproducts. J Neural Transm. 1998;105(4-5):439-61. |
|
|
 |
Sasaki N, Toki S, Chowei H, et al. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Res. 2001 Jan 12;888(2):256-62. |
|
|
 |
Dukic-Stefanovic S, Schinzel R, Riederer P, Munch G. AGES in brain ageing: AGE-inhibitors as neuroprotective and anti-dementia drugs? Biogerontology. 2001;2(1):19-34. |
|
|
 |
Moreira PI, Smith MA, Zhu X, et al. Oxidative stress and neurodegeneration. Ann NY Acad Sci. 2005 Jun;1043:545-52. |
|
|
 |
Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000 Jun 15;28(12):1708-16. |
|
|
 |
Schleicher E, Weigert C, Rohrbach H, et al. Role of glucoxidation and lipid oxidation in the development of atherosclerosis. Ann NY Acad Sci. 2005 Jun;1043:343-54. |
|
|
 |
Asif M, Egan J, Vasan S, et al. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA. 2000 Mar 14;97(6):2809-13. |
|
|
 |
Spiteller G. Is atherosclerosis a multifactorial disease or is it induced by a sequence of lipid peroxidation reactions? Ann NY Acad Sci. 2005 Jun;1043:355-66. |
|
|
 |
Athar M. Oxidative stress and experimental carcinogenesis. Indian J Exp Biol. 2002 Jun;40(6):656-67. |
|
|
 |
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004 Nov;266(1-2):37-56. |
|
|
 |
Abe R, Shimizu T, Sugawara H, et al. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J Invest Dermatol. 2004 Feb;122(2):461-7. |
|
|
 |
Yamamoto Y, Yamagishi S, Hsu CC, Yamamoto H. Advanced glycation endproducts-receptor interactions stimulate the growth of human pancreatic cancer cells through the induction of platelet-derived growth factor-B. Biochem Biophys Res Commun. 1996 May 24;222(3):700-5. |
|
|
 |
van Heijst JW, Niessen HW, Hoekman K, Schalkwijk CG. Advanced glycation end products in human cancer tissues: detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Ann NY Acad Sci. 2005 Jun;1043:725-33. |
|
|
 |
Boldyrev AA, Stvolinsky SL, Tyulina OV, et al. Biochemical and physiological evidence that carnosine is an endogenous neuroprotector against free radicals. Cell Mol Neurobiol. 1997 Apr;17(2):259-71. |
|
|
 |
Wang AM, Ma C, Xie ZH, Shen F. Use of carnosine as a natural anti-senescence drug for human beings. Biochemistry (Mosc.). 2000 Jul;65(7):869-71. |
|
|
 |
Yuneva MO, Bulygina ER, Gallant SC, et al. Effect of carnosine on age-induced changes in senescence-accelerated mice. J Anti-Aging Med. 1999;2(4):337-42. |
|
|
 |
Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12(20):2293-315. |
|
|
 |
Hipkiss AR, Michaelis J, Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995 Aug 28;371(1):81-5. |
|
|
 |
Hipkiss AR. Carnosine, a protective, anti-ageing peptide? Int J Biochem Cell Biol. 1998 Aug;30(8):863-8. |
|
|
 |
Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry (Mosc.). 2000 Jul;65(7):866-8. |
|
|
 |
Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci USA. 1988 May;85(9):3175-9. |
|
|
 |
Hipkiss AR, Preston JE, Himswoth DT, Worthington VC, Abbot NJ. Protective effects of carnosine against malondialdehyde-induced toxicity towards cultured rat brain endothelial cells. Neurosci Lett. 1997 Dec 5;238(3):135-8. |
|
|
 |
Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN. Toxic effects of beta-amyloid(25-35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and beta-alanine. Neurosci Lett. 1998 Feb 13;242(2):105-8. |
|
|
 |
Jandeleit-Dahm KA, Lassila M, Allen TJ. Advanced glycation end products in diabetes-associated atherosclerosis and renal disease: interventional studies. Ann NY Acad Sci. 2005 Jun;1043:759-66. |
|
|
 |
Liu J, Masurekar MR, Vatner DE, et al. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am J Physiol Heart Circ Physiol. 2003 Dec;285(6):H2587-91. | |
| | |
|