|
hc8meifmdc|20005939267D|healthm_live|health_library|health_library_details|0xfdffc1bd010000009901000001001900
| Feel Better with Phenylalanine |
| |
 |
Phenylalanine
May Cheer You Up
It seems to improve mood in depressed patients
by conversion to the “love molecule,†phenylethylamine
By Will Block
We all want happiness, right? And the Declaration of Independence cites our “unalienable Right†to pursue it. Some people choose to do that with mind-altering drugs, some of which can also alter the structure of the brain-and not in a good way. The brain is an organ so exquisitely fine-tuned to perform its innumerable tasks-among which is to make us feel happy-that disrupting its delicate chemical balances with molecules that are alien to it is foolhardy. | |
|
Depression Is Still a Challenge
Of course, those chemical balances can also be disrupted in the normal course of events, and that’s bad enough. Sometimes we’re not happy, and if that condition is severe enough and lasts long enough, it’s called depression.
A susceptibility to depression has hung like a black cloud over mankind throughout history. During that time, men and women have applied endless ingenuity toward finding remedies that work. Some of them do work-sometimes, for some people. It’s an iffy proposition, and the challenge remains almost as great as ever. The advent of antidepressant drugs in modern times has been an undeniable blessing, but it’s one with some unpleasant strings attached.
Enter Phenylalanine
A more natural approach is to use nutritional supplements, which many people prefer because they’re largely free of adverse side effects, and they’re less expensive too. The amino acid phenylalanine has long been of interest because of its role in the production of dopamine and noradrenaline, two neurotransmitters that play key roles in the regulation of mood, especially with regard to our sense of well-being, i.e., our happiness. Significantly, deficiencies of these neurotransmitters in the brain are associated with depression.
Phenylalanine is an essential amino acid-it must be obtained from outside sources because our bodies cannot synthesize it in appreciable amounts. It’s one of the 20 common amino acids found in dietary proteins that we obtain from plants and animals. (Babies, take note: breast milk is particularly rich in phenylalanine.)
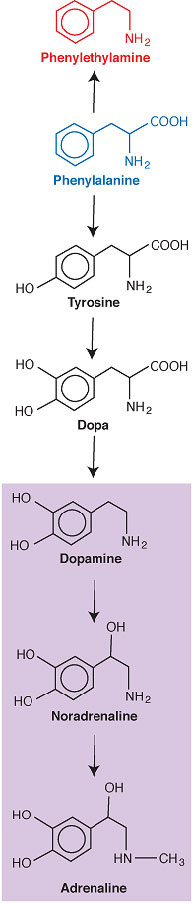
Two major metabolic pathways of phenylalanine: via tyrosine and dopa to the catecholamines (purple tint) or to phenylethylamine.
How Amino Acids Are Used
|
 |
When our digestive juices degrade dietary proteins to their constituent amino acids, the latter enter our bloodstream and then enter the cells of our bodies, there to be used in different ways. The main use is in the synthesis of new (human) proteins, the workhorses of life processes. Amino acids also undergo chemical degradation to form major metabolic intermediates that can be converted to glucose (our premier cellular fuel) or oxidized in the Krebs cycle, the biochemical pathway that provides the chemical energy for life.
Also important is the use of amino acids as the chemical precursors of many small molecules with diverse and biologically important roles. Among these derivatives are purines and pyrimidines, the organic bases that constitute the base pairs (the “rungs in the ladderâ€) in the structure of our nucleic acids, DNA and RNA.
Some amino acids also serve as precursors for hormones. In our adrenal glands, for example, some tyrosine-another amino acid that’s found in our food-is converted to a nonfood amino acid called dopa (aka L-dopa or levodopa). Some dopa, in turn, is converted to the hormone dopamine, some of which is converted to the hormone noradrenaline, some of which is converted to the hormone adrenaline. These three hormones are called catecholamines because their molecular structures incorporate that of the compound catechol.*
*For two previous articles on this subject, see “Catecholamines Kick Out the Demons of Depression†(September 2003) and “Nourish Your Brain with Amino Acids†(September 2004).
Why Tyrosine Is Important
The catecholamines are hormonal neurotransmitters in both the peripheral and central nervous systems.†The same biochemical pathway that converts tyrosine to the catecholamines in the adrenal glands occurs in the brain, albeit under different conditions and with different consequences. Since the catecholamines are not produced via any other pathway, the key compound, obviously, is tyrosine, which is found in abundance | |
| Two major metabolic pathways of phenylalanine: via tyrosine and dopa to the catecholamines (purple tint) or to |
| |
|
in our food (especially cheese). That means that our catecholamine levels depend entirely on tyrosine, right?
†Compared with dopamine and noradrenaline, adrenaline is rather unimportant as a neurotransmitter. It’s enormously important, however, as a hormone that affects various processes in the body, especially those involved in cardiovascular function. By the way, the terms adrenaline and noradrenaline are obsolete among most scientists, who prefer to call them epinephrine and norepinephrine. We use the former terms because they’re more familiar to laymen.
Wrong. In addition to being obtained from food, small amounts of tyrosine are produced in our bodies by the conversion of phenylalanine to tyrosine. Phenylalanine levels, therefore, have some impact on tyrosine levels and hence on catecholamine levels. But how great an impact? That’s hard to say, because both phenylalanine and tyrosine also participate in multiple biochemical pathways that are unrelated to the catecholamines. Depending on the circumstances, they can go off in different directions, including those outlined above for amino acids in general.
The Complexity . . . The Ambiguity . . .
|
 |
Think of these metabolic pathways as a complex of intersecting freeways with multiple cloverleaf formations and on and off ramps for funneling traffic in every possible direction. Over time, the traffic in any given section of the system will ebb and flow, varying from sparse to congested (or gridlocked). Trying to predict the ever-changing patterns throughout the system is very difficult, because it depends on constantly changing circumstances and intricate, multilayered feedback loops, both positive and negative.
And that task is easy compared with trying to understand and predict neurochemical behavior via multiple networks of interlocking metabolic pathways: the math is complex, and even with the requisite deep knowledge of biochemistry and physiology, it can be very confusing-as are the results of numerous studies in this area. Where the effects of phenylalanine and tyrosine on mood are concerned, the data have been ambiguous and often contradictory. | |
| In general, it appears that tyrosine, even though it increases the levels of dopamine and noradrenaline in the blood and the brain, has little or no effect on mood in healthy people. It may, however, be helpful in those who are suffering from depression or who have a history of depression. Curiously, it seems to have an effect on neurons that are sensitive to catecholamines only when the neurons have been very active, but not otherwise.1 |
| |
|
Phenylethylamine-The Hidden Asset
It’s significant that the administration of supplemental dopa, which lies between tyrosine and dopamine in the metabolic pathway, produces no antidepressant effects. This suggests that supplemental tyrosine may exert its effects not via dopa and the catecholamines, but instead via its conversion in the opposite direction, to phenylalanine (this is allowed by the laws of chemistry).2
But how could phenylalanine exert antidepressant effects? Well, phenylalanine is the precursor to a psychoactive compound you may have heard of: phenylethylamine, aka the “love molecule.†In the brain, phenylethylamine (PEA for short) acts as a neuromodulator-a compound that influences the actions of neurotransmitters-in this case, dopamine and noradrenaline.
Thus, even if phenylalanine and tyrosine don’t affect the levels of dopamine or noradrenaline via the tyrosine pathway-and we don’t know for sure whether they do or not-they may indirectly affect the activity of these neurotransmitters via the PEA pathway.3 (Perhaps both mechanisms are involved.)
Although plasma levels of phenylalanine and PEA are correlated, dietary intake of phenylalanine appears to have no short-term (overnight) effect on PEA levels.4 This is probably a reflection of the multiple metabolic pathways that phenylalanine can take, which dilute its short-term effects on any given metabolite. In the longer term, however, the effect of phenylalanine on PEA levels can be seen.
Of Romance and Chocolate
In the laboratory, PEA is the precursor to a great variety of other psychoactive compounds, including neurotransmitters, hormones, stimulants, antidepressants, and hallucinogens. One such derivative is amphetamine, and PEA’s pharmacological properties are, in fact, similar to those of amphetamine.5 (Remember, though, that PEA is made naturally in the brain and elsewhere in the body, in small, safe quantities.)
PEA has been linked neurologically with the euphoria of the early stages of romantic love (hence the nickname), and it’s found, perhaps not coincidentally, in chocolate. (It’s also found in oil of bitter almonds, which is not quite as popular as chocolate on Valentine’s Day.) This discovery led to the “chocolate theory of love,†but the theory doesn’t hold much water, alas, because dietary PEA is so quickly metabolized by the enzyme monoamine oxidase-B in the blood that hardly any of it can get through to the brain.*
Be Positive!
A very important part of being happy-and healthy-is to maintain a positive attitude, no matter what. (Remember Norman Vincent Peale?) That’s easier said than done, of course, and if you could use a little help along the way, a phenylalanine formulation might be just the thing to lift your spirits. If it works for you, that’s great. Scientists would love to know exactly how it works. But it doesn’t really matter, does it?
References
|
 |
Milner JD, Wurtman RJ. Catecholamine synthesis: physiological coupling to precursor supply. Biochem Pharmacol 1986;35(6):875-81. |
|
|
 |
Kravitz HM, Sabelli HC, Fawcett J. Dietary supplements of phenylalanine and other amino acid precursors of brain neuroamines in the treatment of depressive disorders. J Am Osteopath Assoc 1984;S4/1Suppl:119-123. |
|
|
 |
Pogson CI, Knowles RG, Salter M. The control of aromatic amino acid catabolism and its relationship to neurotransmitter amine synthesis. Crit Rev Neurobiol 1989;5(1):29-64. |
|
|
 |
Davis BA, O’Reilly RL, Placatka CL, Paterson A, Yu PH, Durden DA. Effect of dietary phenylalanine on the plasma concentrations of phenylalanine, phenylethylamine, and phenylacetic acid in healthy volunteers. Prog Neuro-Psychopharmacol Biol Psychiat 1991;15:611-23. |
|
|
 |
Janssen PA, Leysen JE, Megens AAHP, Awouters FHL. Does phenylethylamine act as an endogenous amphetamine in some patients? Int J Neuropsychopharmacol 1999;2:229-40. | |
| |
|
A Tale of Two Studies
Two studies conducted several decades ago provided some clinical evidence of a benefit of phenylalanine in depressed patients. In the first study, German researchers tested 20 adult patients who had been diagnosed with various types of depression.1 The study was open-label, i.e., the patients knew they were receiving phenylalanine; there was no placebo control.
For 20 days, the patients received 75 mg of phenylalanine per day, except for three patients who received 200 mg/day. At the end of the test period, 8 patients were completely recovered, and 4 had shown a good response to the treatment. Another 4 were only mildly to moderately improved, and 4 showed no improvement at all.
The researchers cited studies showing that depression is associated with reduced urinary excretion of phenylethylamine, whereas mania and schizophrenia are associated with its increased excretion. They speculated that the positive results seen in their study could be attributed to the metabolism of phenylalanine to phenylethylamine.
In the second study, researchers in Chicago recruited 40 adult patients with major depressive disorders, for an open-label study that lasted 3 or more weeks for each patient.2 The patients were given daily doses of phenylalanine that started out at 1200 mg (1.2 g) and increased to a maximum of 14 g. At the end of the trial, 11 of the patients had recovered completely, 20 had shown partial recovery, and 9 had been unaffected by the treatment.
Here too, the researchers cited prior evidence in support of the phenylethylamine hypothesis to explain their results, and they offered detailed arguments of their own to back that up. They concluded by stating,2
The observed mood-elevating effect of LPA [L-phenylalanine] complements a series of observations suggesting that low PEA [phenylethylamine] levels may underlie some forms of depression and that the brain is able to use dietary amino acids to enhance the production of brain neuroamines capable of sustaining mood.
References |
 |
Beckmann H, Strauss MA, Ludolph E. DL-Phenylalanine in depressed patients: an open study. J Neural Transmission 1977;41:123-34. |
|
|
 |
Kravitz HM, Sabelli HC, Fawcett J. Dietary supplements of phenylalanine and other amino acid precursors of brain neuroamines in the treatment of depressive disorders. J Am Osteopath Assoc 1984;S4/1Suppl:119-123. | |
| | |
|